At the heart of many high-performance coatings lies a manufacturing process known as electrodeposition, or electroplating. Traditionally driven by direct current (DC), this method has met limitations when trying to achieve uniform nanostructured alloys. To overcome these challenges, Xtalic leverages pulse current and pulse reverse plating techniques to engineer advanced coatings with unmatched control over structure and performance. Let’s take a look at how it can be used in electroplating of innovative metallic alloys.
Contents
Electroplating Alloys with Different Deposition Potentials
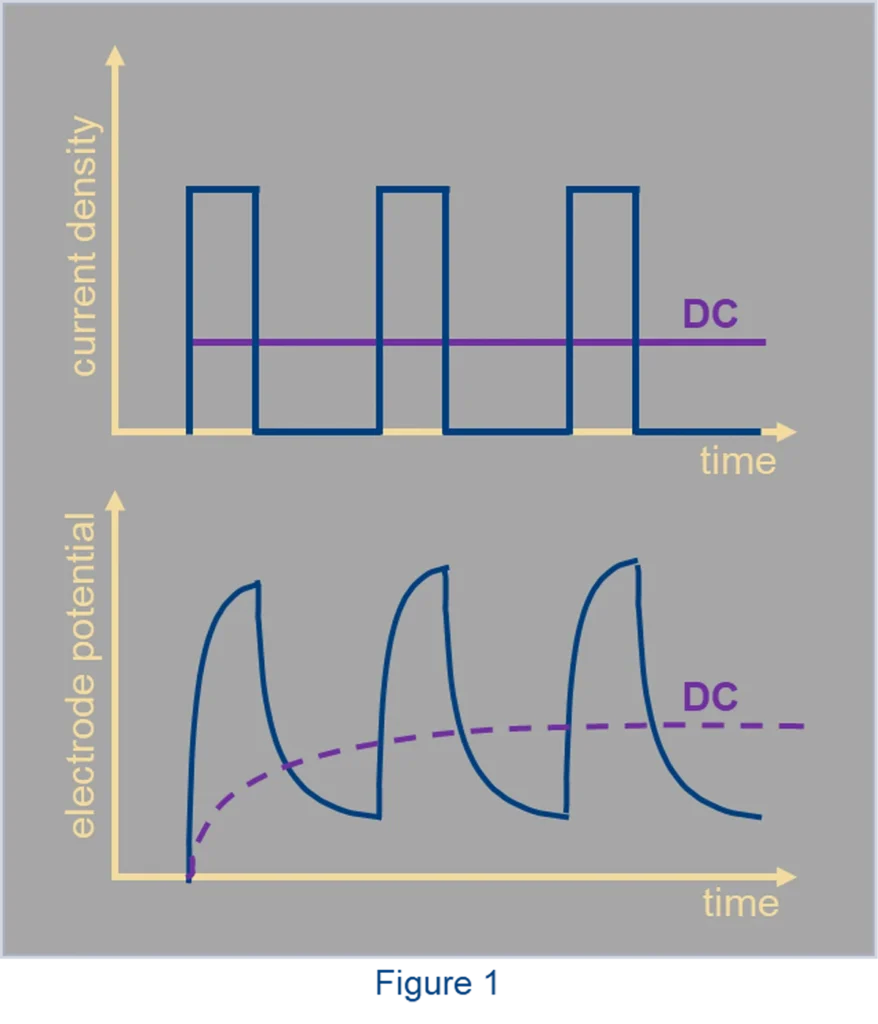
Direct current (DC) creates a certain potential on the cathode surface. To obtain an alloy, this potential needs to be more negative than the deposition potentials of both metals. Sometimes, to reach such a potential, very high current density needs to be applied, which may lead to fast plating and undesirable side reactions. A pulse current with high amplitude and short duty cycle would create negative potential for a very short time, and the following rest period can be adjusted so that the average current density is low. A DC equal to this average would not have yielded the desired alloy. The figure below shows pulse waveforms in terms of current density and electrode potential along with their average DC equivalents.
Overcoming Phase Segregation in Alloy Electroplating
Typically, concentrations of alloying elements in the electrolyte are not the same as their concentrations in the plated alloy. In many cases it is not an issue, but in some systems, it leads to depletion of one of the elements near the cathode surface. It may result in a non-uniform speciation of metallic ions in the vicinity of the electrode and a two-phase deposit: low alloy and high alloy areas. Using short pulses with long rest times helps even out the electrolyte before the next pulse is applied.
A stark example of this phenomenon can occur in the electrodeposition of aluminum-zirconium alloys from ionic liquids.
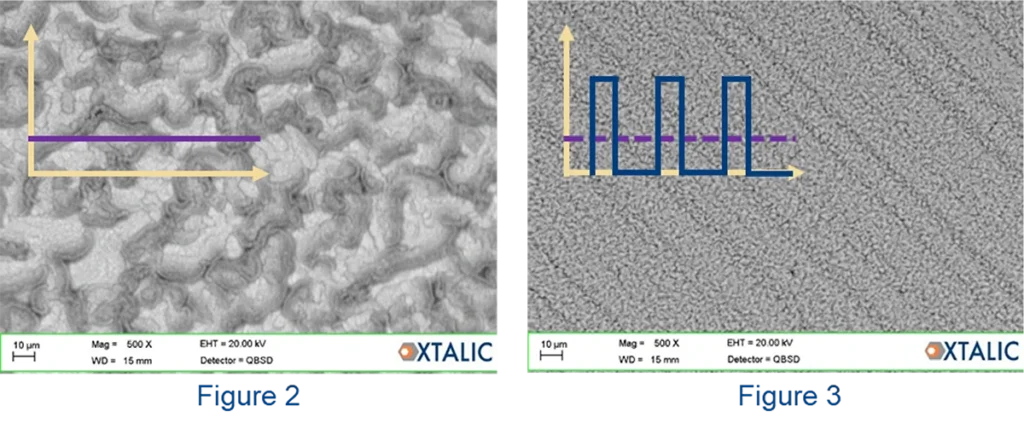
Al-Zr plated by DC current (Fig.2) has distinct areas of low Zr (dark zones, ~5% Zr) and high Zr (light zones, up to 12% Zr). Applying very fast and short pulses with longer off times enables more uniform alloys at the same average current density (Fig.3) and the same average composition of 7% zirconium.
Expanding the Process Window in Electroplating with Current Modulation
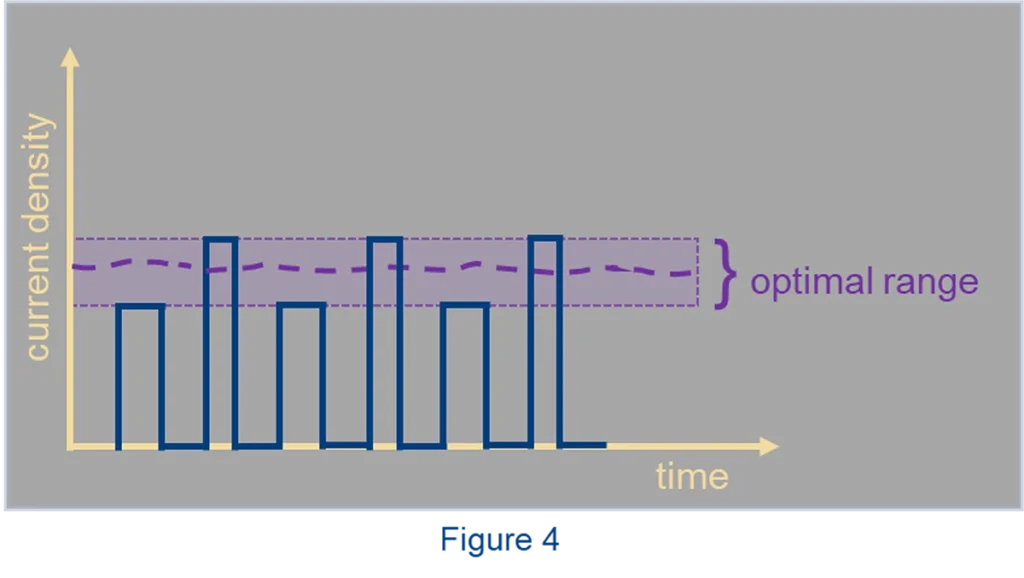
Some solute alloying elements are rather difficult to co-deposit together with the solvent metal of the alloy. The amount of the solute element is low and might go through a maximum at a particular current density. The operating window becomes narrow, which makes it hard to transition from one plating line to another or to plate samples of various shapes and sizes. Here a current waveform with several pulses of various amplitudes can be applied, some currents below the maximum and some above. If the exact maximum has moved because of a slight change in conditions, like flow or temperature, we can still capture the desired regime by current pulses around the optimal point (Fig. 4). This more complex waveform creates an opportunity to homogenize the alloy and expand the operating window.
Enhancing Alloy Composition with Pulse Reverse Electroplating
A pulse/reverse waveform gives even more flexibility to the process of electrodeposition. This method allows an opportunity to engineer the alloy composition in the case when anodic current selectively dissolves one of the elements, and not the other. In an ideal reversible system, the more electrochemically negative of the two elements plates last and dissolves first. However, in the real electrolyte, there are factors other than potential, affecting the relative deposition and dissolution rate of an element. For instance, under anodic bias one of the alloying constituents may form a protective oxide layer that is hard to dissolve, or it may catalyze a side reaction instead of dissolving itself. Including anodic pulses in the waveform can be used to increase the concentration of such an element in the alloy.
Xtalic’s Expertise in Custom Electroplating Solutions
Xtalic’s proprietary alloy design and electrochemical expertise are the foundation of our ability to deliver mission-critical materials. Our pulse current plating waveforms are tailored to achieve nanostructures that outperform conventional materials in durability, conductivity, and corrosion resistance. Pulse current and pulse reverse plating are powerful tools in the evolution of materials science. By moving beyond the limits of DC plating, Xtalic engineers precision coatings that enable new product features, improve reliability, and reduce environmental impact. If your products demand next-level performance, Xtalic has the expertise to deliver the advanced nanostructured materials you need.
Contact Us
Connect with Xtalic to learn how our pulse engineered alloys can help you overcome your toughest materials challenges.
"*" indicates required fields