A review of our time at The Electrochemical Society Conference with electrochemistry experts
As a company focused on applied innovation, we always have an eye out for the latest in electrochemistry technology and science fundamentals that have a pathway to commercialization. R&D scientists need to have the absorptive capacity[1] to see a graduate student paper and visualize how this might be useful in a commercial application. Technical conferences are a great source of farming for new ideas, networking, and applying our absorptive capacity.
At the ECS – The Electrochemical Society Conference last week, conveniently located right here in Boston, Xtalic sought to both teach and learn from the experts in electrochemistry who gathered from around the world.
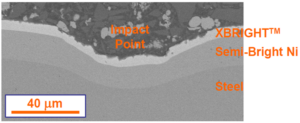
An application of nanocrystalline Ni-W (XBRIGHT) showing a cross-section micrograph of an impact test in a plated steel part where the plating survives a challenging test without cracking or peeling. (Image: Tom Clay, Xtalic)
Xtalic CEO Tom Clay presented the case study of the challenges in commercializing hard technology. We need to find a durable value proposition for each new research project and application. Tom walked us through the history of Xtalic applications in the style of a Harvard Business School review.
Bob Hilty followed up with a talk discussing the linkage between MIT and Xtalic and how to implement and scale the fundamental science at MIT into real and valuable products here at Xtalic.
David Bruce, a Professor at the University of Ottawa, spoke passionately about the importance of integrating environmental stewardship into our product and process design. This follows a common theme, more often seen in government-subsidized programs, to focus on the global impact of our project choices.
The environmental themes continued, next up from Italy with a talk by Massimo Innocenti from Università degli Studi di Firenze. They noted the importance of reducing precious metals from designs, which is obvious from a cost perspective, but there is a more pressing need to reduce the safety hazards of electroplating through the elimination of cyanide-containing electrolytes and hazardous materials. While these have always been desirable traits, the need for new formulations is becoming more urgent, and we are seeing investment dollars flow here.
Sometimes a researcher finds a way to turn a bug into a feature. When we very rapidly plate some materials like copper, the resulting deposit is dendritic and porous.

Copper film before and after compression. (Image: Jean-Yves Hihn)
Jean-Yves Hihn from the University of Franche Comte leveraged this effect to build 50um thick highly porous copper sponge. This sponge can then be compressed between two plates to form a reliable one-time-use interconnect. The copper sponge is crushed and collapsed during mating, accommodating any mismatch in coplanarity or roughness between the mating interfaces. While the thermal conductivity was substantially less than pure copper, the technology showed the ability to make a robust connection.
Lastly, there were talks about metals reclamation and sourcing. Rare earth metals have become a hotbed of activity with US government investment in the search for alternatives or additional sources for these needed materials. Researchers from Case Western Reserve University presented their plan to recycle Nd metals from recycled magnet sources using an electrowinning approach and molten salt electrolytes. This approach was novel in that it avoids fluorine-containing molten salts and the associated environmental hazards. Apple has demonstrated its robotic disassembly approaches to harvesting materials from old model iPhones, which could couple with this approach to reclaim the magnetic materials.
These few highlights from ECS help to paint some direction in our research pipeline and feed our creativity.
Thoughts?
[1] Absorptive capacity has been studied by many but one of the earlier articles by Cohen and Levinthal is recommended reading.